Lyka Smith our proud to be the Australian Distributor of Su-Por stock and custom implants by Poriferous.
Su-Por Surgical Implants are long-term implantable medical devices for the augmentation and restoration of the craniomaxillofacial area. Poriferous produce them from pure porous high-density polyethylene, the gold standard of polymer biocompatibility.
The devices are available in sheets, blocks, spheres, and anatomical shapes. The porous structures’ unique nature allows for the patients’ own fibrovascular to integrate, eliminate migration, reduce infection, and improve clinical performance.
Unlike non-porous implants, Su-Por biomaterial allows the patients’ immune system to effectively prevent and eliminate potential infections around and throughout the porous structure. Clinical evidence of Su-Por has proven to be superior to non-integrating implants. It is often cited as the ideal material for notoriously difficult revision procedures where non-porous devices required removal, often leaving damaged and scarred tissue.
Choosing the right surgical implant is essential for ensuring safe and successful patient outcomes. That’s why Su-Por Surgical Implants, manufactured and supplied by Poriferous, LLC, are the ultimate choice for surgical procedures that require life-lasting and highly customizable implants.

Lyka Smith are proud to supply Patient Specific Su-Por Implants.
These implants are designed using the patient’s own anatomy to provide optimal reconstruction and long term results.
Poriferous are also a world first manufacturer of Patient Specific Ear Implants.
Patient Specific Su-Por Implants+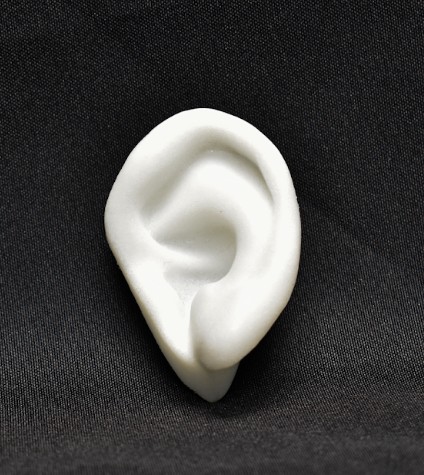
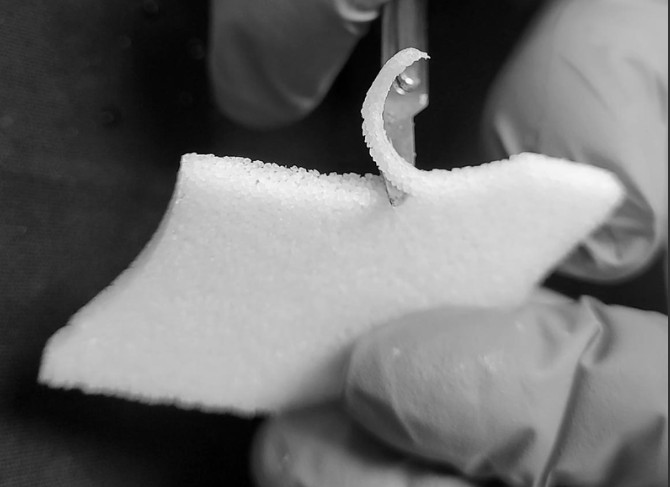
While both implants are made from the same material (porous linear high-density polyethylene (HDPE)) and are used for the same type of surgeries, Su-Por Surgical Implants are manufactured using proprietary technology that, according to surgeon feedback, yields a higher quality result.
In addition, the Poriferous design and production team has over 70 years of combined experience working with porous polyethylene (HDPE) implants. This experience exceeds that of all other porous polyethylene manufacturers in the world.
The key reasons why Su-Por Surgical Implants are more flexible than other surgical Implants are the proprietary processes, the consistency with which the implants are made, and the key design elements. Based on the experience of members of the Poriferous team, formerly involved in the manufacture of other implants, and feedback from surgeons who have used both Su-Por and other implants, Su-Por Surgical Implants are more flexible and less likely to break when manipulated.
While polyethylene has been used as an implant material since the 1940’s, and porous polyethylene has been used since the mid 1970’s, Su-Por was introduced to market in 2014.
Su-Por patients experience low complication rates. For more information on the clinical performance of Su-Por see the clinical data document.
Lyka Smith will provide our instrumentation sets with all Su-Por orders where required.
Yes all Su-Por implants are sent in sterile packaging.